|
Molecular/Electronic Structure |
Oxidation/Reduction |
Volume Change Mechanism |
PPy (DBS) |
Further Information
Conjugated polymers (also known as conducting polymers) are distinguished
by alternating single and double bonds between carbon atoms on the polymer
backbone. The conjugated polymer with the simplest chemical structure is
polyacetylene, shown here:
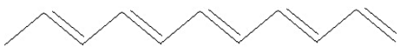
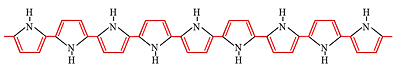
The polymer we use for our microactuators is polypyrrole (PPy), shown
with the conjugation path highlighted in red.
Conjugated polymers are organic semiconductors. This means that, like
silicon, they have a band gap.
- They can emit light, the color of which is tailorable through the
chemical structure, which may find commercial applications in light emitting
diodes (LEDs) and displays (although this technology competes with OLEDs,
or organic LEDS, which use small molecules).
- Electron-hole pairs are generated upon absorbing light, and so these
materials can be used in photovoltaic devices.
Conjugated polymers can be “doped”, which changes their properties. They are usually p-doped, or oxidized. Changing the oxidation level of a conjugated polymer means changing the number of electrons on its backbone. Oxidation means removing electrons, reduction means adding electrons. This process can be done electrochemically, and the potential difference between oxidized and reduced states is usually less than 1 V. The oxidation level varies continuously and smoothly (i.e. in an analog way) between the fully oxidized and fully reduced states and can be held fixed at any state by the appropriate potential. When the oxidation level is varied, many of the properties of the material change, including its
- electrical conductivity
- volume (this leads to application in actuators)
- color (see the section Moving Electrochromic Device on Silicon; this leads to applications in electrochromic windows and displays; )
- mechanical properties (such as the Young’s modulus and tensile strength)
- hydrophobicity
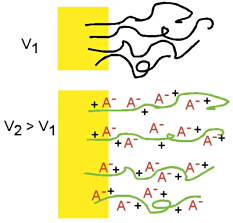
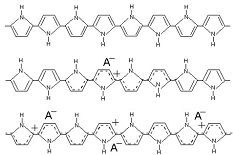 This is a schematic illustration of the oxidation process in polypyrrole, going from the neutral, insulating state (top) to a partially oxidized state (middle) to the fully oxidized conducting state (bottom). To maintain charge neutrality, an anion is inserted into the material for each positive charge on the chain. Fully doped, the dopant concentration in PPy is approximately 1 dopant for every 3-4 pyrrole rings. (This example is for a small, mobile anion.) This is a schematic illustration of the oxidation process in polypyrrole, going from the neutral, insulating state (top) to a partially oxidized state (middle) to the fully oxidized conducting state (bottom). To maintain charge neutrality, an anion is inserted into the material for each positive charge on the chain. Fully doped, the dopant concentration in PPy is approximately 1 dopant for every 3-4 pyrrole rings. (This example is for a small, mobile anion.) |
|
In this text, the oxidized state will be represented schematically as:
|
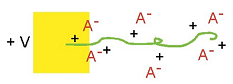
 Volume change occurs primarily because of the insertion of ions, as illustrated schematically here (again assuming a mobile anion). Anions are inserted during oxidation, and they take up space in the polymer. The ions are usually solvated, or surrounded by solvent molecules (not shown), and this solvation shell can be the dominant contributor to the effective volume of the ion. Volume change occurs primarily because of the insertion of ions, as illustrated schematically here (again assuming a mobile anion). Anions are inserted during oxidation, and they take up space in the polymer. The ions are usually solvated, or surrounded by solvent molecules (not shown), and this solvation shell can be the dominant contributor to the effective volume of the ion. |
|
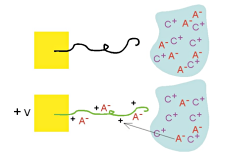
Where do the ions come from? An electrolyte (salt solution) in contact with the polymer. |
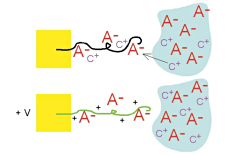 In the prior figures, we assumed the anion was mobile, but if the anions are large, then they can be immobile, or trapped, in the polymer. In that case, cations enter and exit the polymer from the electrolyte instead. The polymer expands during reduction and contracts during oxidation, the opposite of what occurs with small, mobile anions. We use PPy doped with dodecylbenzenesulfonate (DBS), a large immobile anion, for our work, and thus we have cation transport and expansion during reduction. In the prior figures, we assumed the anion was mobile, but if the anions are large, then they can be immobile, or trapped, in the polymer. In that case, cations enter and exit the polymer from the electrolyte instead. The polymer expands during reduction and contracts during oxidation, the opposite of what occurs with small, mobile anions. We use PPy doped with dodecylbenzenesulfonate (DBS), a large immobile anion, for our work, and thus we have cation transport and expansion during reduction.
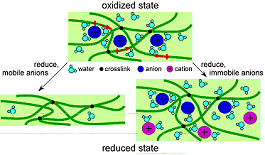 In summary: It is important to emphasize that in a partially oxidized or reduced state, the volume is intermediate between the minimum and maximum (this is shown in the sequence of photos in Moving Silicon Plates). The strains (change in length over initial length) in PPy(DBS) are ~3% percent inplane and ~30% out of plane, depending on the cation. Strains in other conjugated polymers is generally similar. Larger strains have been demonstrated, but one generally sacrifices force in that case. In summary: It is important to emphasize that in a partially oxidized or reduced state, the volume is intermediate between the minimum and maximum (this is shown in the sequence of photos in Moving Silicon Plates). The strains (change in length over initial length) in PPy(DBS) are ~3% percent inplane and ~30% out of plane, depending on the cation. Strains in other conjugated polymers is generally similar. Larger strains have been demonstrated, but one generally sacrifices force in that case.
The electrochemical processes can be represented by:
| |
(1) |
P+(A-) + C+ + e- àß P°(AC) |
| |
(2) |
P+(A-) + C+ + e- àß P° + A- + C+ |
where P+ represents the doped (oxidized) state of the polymer and P° the undoped (reduced, neutral) state. P+(A-) indicates that the anion A- is incorporated in the polymer as a dopant. In the case of a large, bulky dopant A-, the anion is immobile and the cation C+ enters the film to maintain charge neutrality upon undoping (reduction), resulting in a volume expansion (eqn. 1). If the anion is small and mobile, it is expelled and a volume contraction results upon reduction (eqn. 2). (Both processes may occur simultaneously for an intermediate-size anion, which makes actuator control almost impossible because the volume change is not monotonic.)
It should be noted that the discussion above has been oversimplified to convey the basic ideas, and interested site visitors should consult the references for more comprehensive and nuanced explanations.
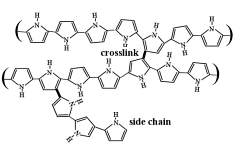 As mentioned above, in our work we use polypyrrole doped with dodecylbenzenesulfonate, PPy(DBS). It is electrochemically deposited, as are virtually all PPy films that are used for actuation. The polymer is deposited in the oxidized state, and thus incorporates dopants (anions) during growth. These dopants strongly affect the properties of the final material. As mentioned above, in our work we use polypyrrole doped with dodecylbenzenesulfonate, PPy(DBS). It is electrochemically deposited, as are virtually all PPy films that are used for actuation. The polymer is deposited in the oxidized state, and thus incorporates dopants (anions) during growth. These dopants strongly affect the properties of the final material.
One of the oversimplifications in the discussion above concerned the structure of PPy, which does not comprise linear chains, but is heavily crosslinked. This crosslinking traps the DBS- within the PPy matrix and thereby makes the material a cation-transporter. (DBS- is mobile in polyaniline that is not crosslinked.)
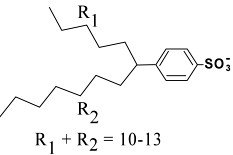
Although the name dodecylbenzenesulfonate would suggest a benzene ring with a sulfonate and a 12-carbon chain, in actual fact the product that one purchases contains a mixture of molecules having 10-13 carbons and mostly branched tails.
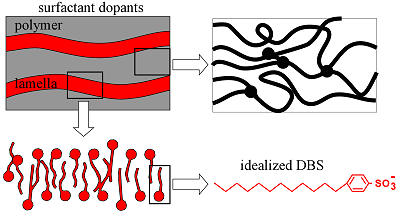
Since DBS- is a surfactant (it has a hydrophilic head group due to the sulfonate and a hydrophobic tail due to the alkyl chains), it imparts a lamellar structure to the PPy, as indicated in this cartoon. (Films deposited with spherical inorganic anions do not have such a structure.) This layered structure, with the layers parallel to the electrode surface, renders PPy(DBS) strongly anisotropic.
For more information see:
E. Smela, "Microfabrication of PPy microactuators and other conjugated polymer devices," J. Micromech. Microeng., 9, 1-18 (1999).
For further information than provided in this simplified overview, please
refer to the following reviews.
Reviews for the lay person
- K. F. Schoch and H. E. Saunders, "Conducting polymers," IEEE
Spectrum, 52-55, (June, 1992).
- P. Yam, "Plastics get wired," Sci. Amer., 74-79, (July, 1995).
- R. B. Kaner and A. G. MacDiarmid, "Plastics that conduct
electricity," Sci. Amer., 60-65, (Feb, 1988).
- M. G. Kanatzidis, "Conductive Polymers," Chem. Eng. News, 36-54, (3 Dec, 1990).
- J. R. Reynolds, "Electrically conductive polymers," Chemtech, 440-447, (July, 1988).
- T. Studt, "Conductive polymers give researchers a big jolt,"
R&D Magazine, 94-96, (Oct, 1991).
- P. S. Zurer, "Plastics with an electrical bent," Chem. Eng. News, 41, (13 Apr, 1998).
Books
- G. G. Wallace, G. M. Spinks, and P. R. Teasdale, Conductive Electroactive Polymers: Intelligent Materials Systems, (Technomic Publishing Co., Lancaster, 1997).
- Handbook of Conducting Polymers, edited by T. A. Skotheim (Marcel Dekker, Inc., New York, 1986).
- Handbook of Conducting Polymers, 2nd ed., edited by T. A. Skotheim, R. L. Elsenbaumer, and J. R. Reynolds (Marcel Dekker, Inc., New York, 1998).
- Handbook of Conducting Polymers, 3rd ed., edited by T. A. Skotheim and J. R. Reynolds (CRC Press, Boca Raton, 2007).
More advanced general reviews
- K. Gurunathan, A. V. Murugan, R. Marimuthu, U. P. Mulik, and D. P. Amalnerkar, "Electrochemically synthesised conducting polymeric materials for applications towards technology in electronics, optoelectronics and energy storage devices," Mater. Chem. Phys., 61 (3), 173-191 (1999).
- A. J. Epstein, "Electrically conducting polymers: science and technology," MRS Bull., June, 16-23 (1997).
- A. G. MacDiarmid, "Synthetic metals: a novel role for organic polymers," Synth. Met., 125 (1), 11-22 (2001).
- A. J. Heeger, "Semiconducting and metallic polymers: the fourth generation of polymeric materials," Synth. Met., 125 (1), 23-42 (2001).
Top of Page
|
 Since DBS- is a surfactant (it has a hydrophilic head group due to the sulfonate and a hydrophobic tail due to the alkyl chains), it imparts a lamellar structure to the PPy, as indicated in this cartoon. (Films deposited with spherical inorganic anions do not have such a structure.) This layered structure, with the layers parallel to the electrode surface, renders PPy(DBS) strongly anisotropic.
Since DBS- is a surfactant (it has a hydrophilic head group due to the sulfonate and a hydrophobic tail due to the alkyl chains), it imparts a lamellar structure to the PPy, as indicated in this cartoon. (Films deposited with spherical inorganic anions do not have such a structure.) This layered structure, with the layers parallel to the electrode surface, renders PPy(DBS) strongly anisotropic.

 This is a schematic illustration of the oxidation process in polypyrrole, going from the neutral, insulating state (top) to a partially oxidized state (middle) to the fully oxidized conducting state (bottom). To maintain charge neutrality, an anion is inserted into the material for each positive charge on the chain. Fully doped, the dopant concentration in PPy is approximately 1 dopant for every 3-4 pyrrole rings. (This example is for a small, mobile anion.)
This is a schematic illustration of the oxidation process in polypyrrole, going from the neutral, insulating state (top) to a partially oxidized state (middle) to the fully oxidized conducting state (bottom). To maintain charge neutrality, an anion is inserted into the material for each positive charge on the chain. Fully doped, the dopant concentration in PPy is approximately 1 dopant for every 3-4 pyrrole rings. (This example is for a small, mobile anion.) 
 Volume change occurs primarily because of the insertion of ions, as illustrated schematically here (again assuming a mobile anion). Anions are inserted during oxidation, and they take up space in the polymer. The ions are usually solvated, or surrounded by solvent molecules (not shown), and this solvation shell can be the dominant contributor to the effective volume of the ion.
Volume change occurs primarily because of the insertion of ions, as illustrated schematically here (again assuming a mobile anion). Anions are inserted during oxidation, and they take up space in the polymer. The ions are usually solvated, or surrounded by solvent molecules (not shown), and this solvation shell can be the dominant contributor to the effective volume of the ion.
 In the prior figures, we assumed the anion was mobile, but if the anions are large, then they can be immobile, or trapped, in the polymer. In that case, cations enter and exit the polymer from the electrolyte instead. The polymer expands during reduction and contracts during oxidation, the opposite of what occurs with small, mobile anions. We use PPy doped with
In the prior figures, we assumed the anion was mobile, but if the anions are large, then they can be immobile, or trapped, in the polymer. In that case, cations enter and exit the polymer from the electrolyte instead. The polymer expands during reduction and contracts during oxidation, the opposite of what occurs with small, mobile anions. We use PPy doped with  In summary: It is important to emphasize that in a partially oxidized or reduced state, the volume is intermediate between the minimum and maximum (this is shown in the sequence of photos in
In summary: It is important to emphasize that in a partially oxidized or reduced state, the volume is intermediate between the minimum and maximum (this is shown in the sequence of photos in  As mentioned above, in our work we use polypyrrole doped with dodecylbenzenesulfonate, PPy(DBS). It is electrochemically deposited, as are virtually all PPy films that are used for actuation. The polymer is deposited in the oxidized state, and thus incorporates dopants (anions) during growth. These dopants strongly affect the properties of the final material.
As mentioned above, in our work we use polypyrrole doped with dodecylbenzenesulfonate, PPy(DBS). It is electrochemically deposited, as are virtually all PPy films that are used for actuation. The polymer is deposited in the oxidized state, and thus incorporates dopants (anions) during growth. These dopants strongly affect the properties of the final material.