Electrochemistry
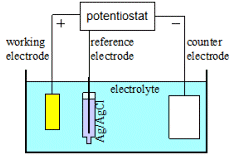
The figure on the left shows a 3-electrode configuration. The applied potential is specified versus the reference electrode, here Ag/AgCl, and the current is supplied by the counter electrode. This is typically used when voltage is controlled; it is unnecessary when controlling current – in that case one can use a 2-electrode configuration with only working and counter electrodes.
|
|
|
©2013
 Electrochemistry uses the transfer of electrons to cause chemical reactions.
Electrochemistry uses the transfer of electrons to cause chemical reactions.